Embark on a scientific odyssey with osmosis and diffusion test questions! This discourse delves into the fundamental principles, influential factors, and practical applications of these vital processes, unveiling their significance in biological systems and beyond.
Osmosis and diffusion, the enigmatic dance of molecules, govern the movement of substances across semipermeable membranes. Understanding their interplay is crucial for unraveling the mysteries of life and technological advancements.
Osmosis and Diffusion: Basic Concepts: Osmosis And Diffusion Test Questions
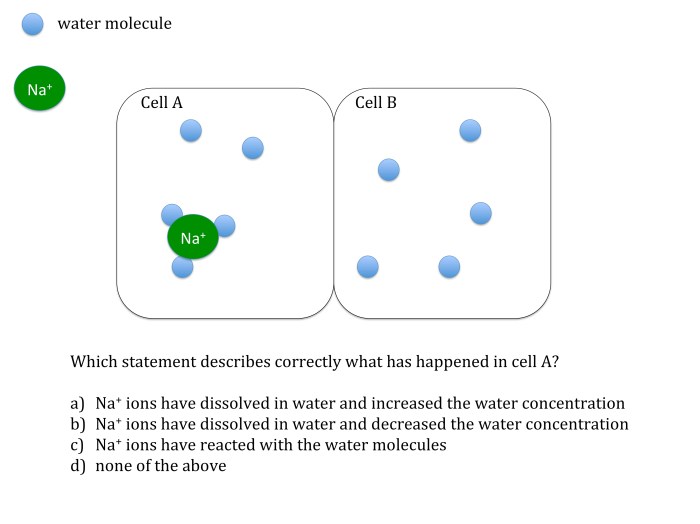
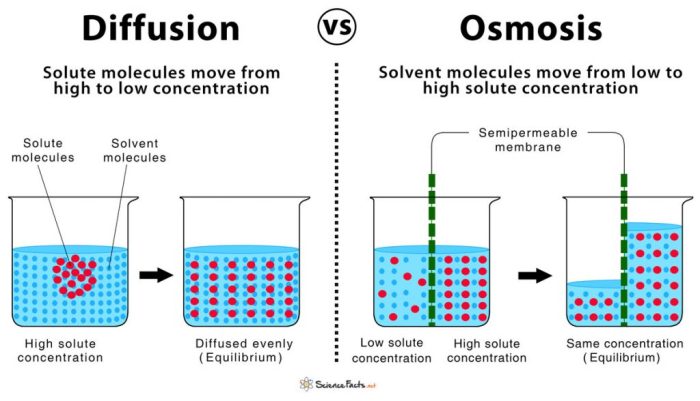
Osmosis and diffusion are two fundamental processes that govern the movement of molecules across semipermeable membranes. Osmosis is the net movement of water molecules from an area of low solute concentration to an area of high solute concentration, while diffusion is the net movement of solute molecules from an area of high concentration to an area of low concentration.
Both processes are driven by the tendency of the system to reach equilibrium, where the concentration of molecules is uniform throughout.
Examples of osmosis and diffusion in everyday life include the absorption of water by plants, the diffusion of oxygen and carbon dioxide in the lungs, and the osmosis of water from the blood into cells.
Key Differences between Osmosis and Diffusion, Osmosis and diffusion test questions
| Characteristic | Osmosis | Diffusion |
|---|---|---|
| Definition | Movement of water molecules | Movement of solute molecules |
| Direction of movement | From low to high solute concentration | From high to low solute concentration |
| Selectivity | Only water molecules can pass through the membrane | Any solute molecules can pass through the membrane |
Clarifying Questions
What is the primary difference between osmosis and diffusion?
Osmosis involves the movement of water across a semipermeable membrane, driven by concentration gradients, while diffusion refers to the movement of particles from an area of high concentration to an area of low concentration.
How does temperature affect the rate of osmosis and diffusion?
Temperature increase typically accelerates the rate of both osmosis and diffusion, as higher temperatures enhance the kinetic energy of molecules, facilitating their movement.
What are some practical applications of osmosis and diffusion?
These processes find applications in water purification, drug delivery, food processing, and industrial separations, among others.